What Happens when 1 + 1 doesn’t equal 2?
In our previous post, we looked at the physical aspect of the volume addition of two different solvents. In this post, we shall review the chemical aspect.
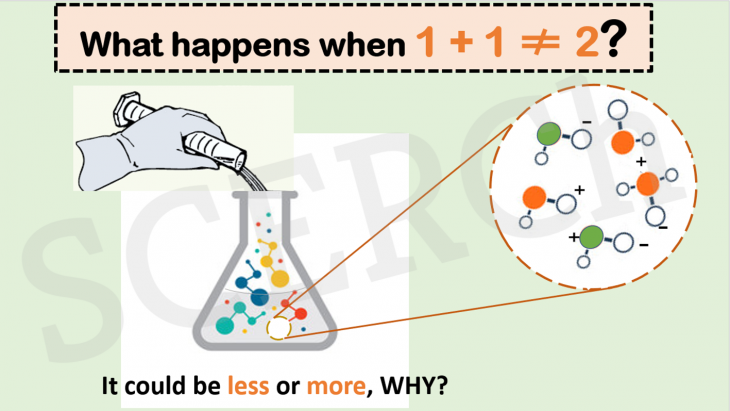
When 2 liquids of equal volumes chemically react to form products. The final volume obtained is dependent on the molecular arrangement of the product molecules. When the same volumes of 2M NaOH is mixed with 2M HCl, the final volume is more than the original volume of the two liquids. How?
The “vanishing volume” or “appearing volume” is due to intermolecular interactions which produces differences in arrangement of the product molecules in the mixture versus the reactant molecules. Strong interactions result in closer packing (vanishing volume) and vise versa.
