Heat Stable Salts (HSSs) Affecting the Foam Tendancy of the Amine Solvent
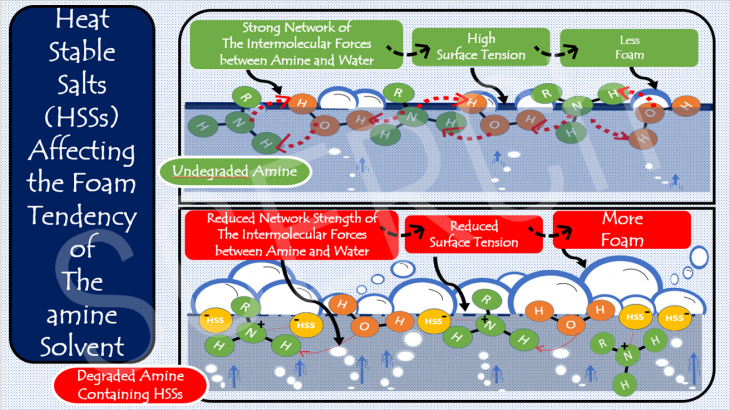
The fundamentals of chemistry can help us to understand why heat stable salts (HSSs) make an amine solution more prone to foaming. Foaming is one of the operational issues that can reduce the ability of an amine to capture the CO2. Foam behaviour of the amine solvent is a manifestation of its surface tension. If we dig deeper into the atomic level, one will find out that the surface tension of the amine is controlled ultimately by the strength of the hydrogen bonding and dipole-dipole forces holding the amine and water molecules together. The principle is that the stronger the forces existing in the solution, the higher its surface tension, and the harder it is for the amine to foam. The strength of the amine-water intermolecular forces changes with HSSs contamination. Once formed from the undesirable degradation of amine, these HSSs instantly tie up the amine molecules which in turn, interrupts the amine-water molecular connectivity. This weakens the original intermolecular forces that exists within the amine solution. This occurrence definitely lowers the amine surface tension which subsequently makes the amine solution more susceptible to foam.
This phenomenon is represented graphically as shown in the figure below. The top picture depicts the scenario in the absence of HSS. The intermolecular forces existing between amine and water are so strong, resulting in a high surface tension, yielding less foaming of the amine. The bottom picture on the other hand illustrates the presence of HSS created as a result of amine degradation. These salts disrupt and break the intermolecular forces that exist between the amine and water, lowering the resistance of the surface forces. As a result, weak intermolecular forces exist between amine and water at the surface, which consequently lowers the surface tension of the amine solution and leads to high foaming. Based on this understanding, a monitoring system that tracks the concentration of HSS must be put in place so as to know when to remove the HSS as their concentration builds up. Apart from using high pressure drop across the absorber tower as a marker for amine foaming, surface tension can also be considered as one of the parameters that can help track the foaming tendency of the amine solution.
