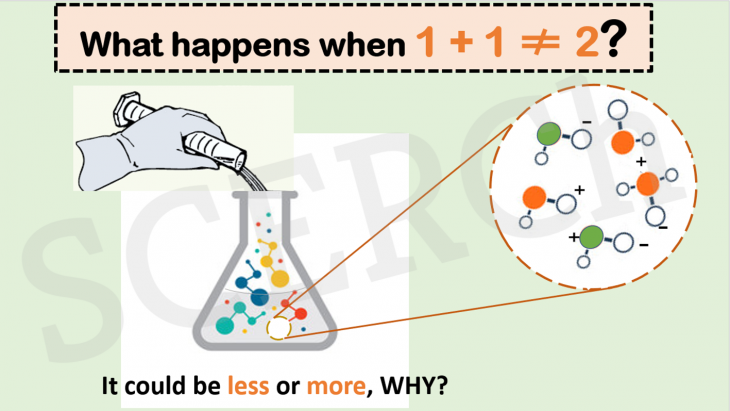
What Happens when 1 + 1 doesn’t equal 2?
In our previous post, we looked at the physical aspect of the volume addition of two different solvents. In this post, we shall review the chemical aspect.
Read More
Did you know that 1 + 1 is not always 2?
When 250 mL of water is added to 250 mL of water or when 250 mL of alcohol is added to 250 mL of alcohol, the final volume will always be 500 mL, as expected. However, when the same water is added to an equal volume of alcohol, the final volume is about 10% less than the original volume of the two liquids. How?
Read More
A Novel Pre-Treatment Process for the Removal of SOx and NOx from Flue Gas
Among the most developed CO2 capture processes is the amine-based chemical absorption process. Nevertheless, implementing the amine-based chemical absorption technology in industrial processes such as fossil fuel power plants, cement production, iron, and steel industries face the challenge of accelerated solvent degradation in the presence of trace components of particulate matter (PM), O2, NOx, and SOx.
Read More
ICLER team visit to KNUST and UMAT Universities of Ghana
Last November, the ICLER program Chair together with some members of the team paid a visit to two universities in Ghana...
Read More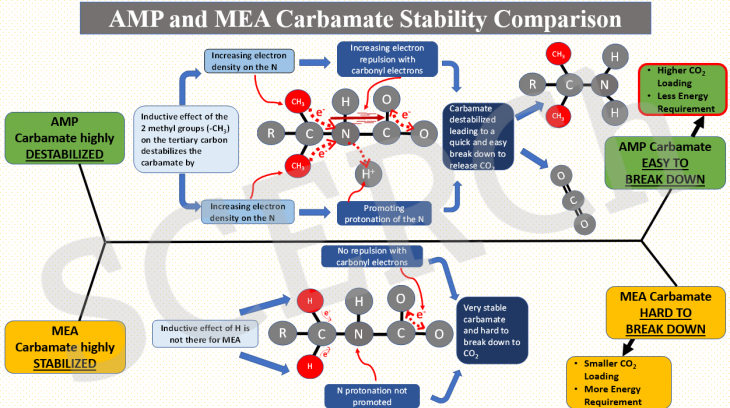
AMP and MEA Carbamate Stability Comparison
In this post, we explain why carbamate of sterically hindered AMP is unstable relative to that of MEA. The instability of AMP carbamate then leads to an easy break down to release CO2 and amine in the desorption process, thus giving rise to a higher CO2 loading and a lower desorption heat requirement than those of MEA.
Read More
Mathematical Modeling, Simulation and Optimization of a CO2 Capture Process in an Absorption Column using a Catalyzed Ionic Solvent as an Absorbent
As the world moves toward a more efficient and cleaner energy ecosystem, research in energy systems improvement and clean fuel technologies have taken the forefront of scientific studies within this domain. However, the development of innovative solutions from the laboratory to an industrial scale can be extremely costly, restricting the breadth of experimentation...
Read More
Choice of Binder in Catalyst Preparation
Catalysts are employed in various industrial applications such as water purification, food, medical, cosmetology, automotive, gas purification, petroleum and precious metal recovery. Catalyst have the ability of speeding up the overall reaction without undergoing any permanent chemical change, as such, most catalyst are expected to be inert. All catalyst consist of an active phase, a support and a promoter or an inhibiter. When it comes to solid catalysts, one more component is key in obtaining the final catalyst; a binder!
Read More
Absorber Column Dimensions
When designing an absorber column to achieve the maximum efficiency, two things need to be determined, the diameter and the height of the column. In lame man’s terms, the diameter refers to how fat the column whereas the height refers to how tall the column will be. For starters, diameter relates to capacity, whereas height relates to the absorption rate...
Read More
Application of CO2 Loaded Ionic Solvent in Concrete
The increase in CO2 emissions, as a major greenhouse gas, has led to increase in average global temperature. At present CO2 levels in the atmosphere are more than 412 ppm and rising. To succeed in limiting the increase in global temperature to 1.5˚ C above pre-industrial levels, CO2 emissions worldwide must be reduced substantially in all sectors of the economy. Carbon capture utilization and storage (CCUS) provides a means of producing low-carbon electricity from fossil fuels and of reducing CO2 emissions from industrial processes such as gas processing, cement and steel making, where other decarbonization options are limited...
Read More
Metal-Organic Frameworks for CO2 Capture
Developing an effective carbon dioxide capture system is essential to reducing greenhouse gas emissions and moving toward a cleaner energy future. As an emerging new class of porous solids, metal-organic frameworks (MOFs) adsorbents are particularly promising as CO2 capture materials because they have high internal surface areas, low heat capacity, and adjustable pore functionality enabling the selective adsorption of large quantities of CO2...
Read More